polar covalent bond
Specifically when the difference in electronegativities of the two atoms in the bond is between 04 and 17. The dipole moment of a molecule is therefore the.
 |
| Polar And Non Polar Covalent Molecules Polar Vs Nonpolar Youtube Playlist Science Chemistry Chemistry Science Activities |
They possess both a magnitude and a direction.
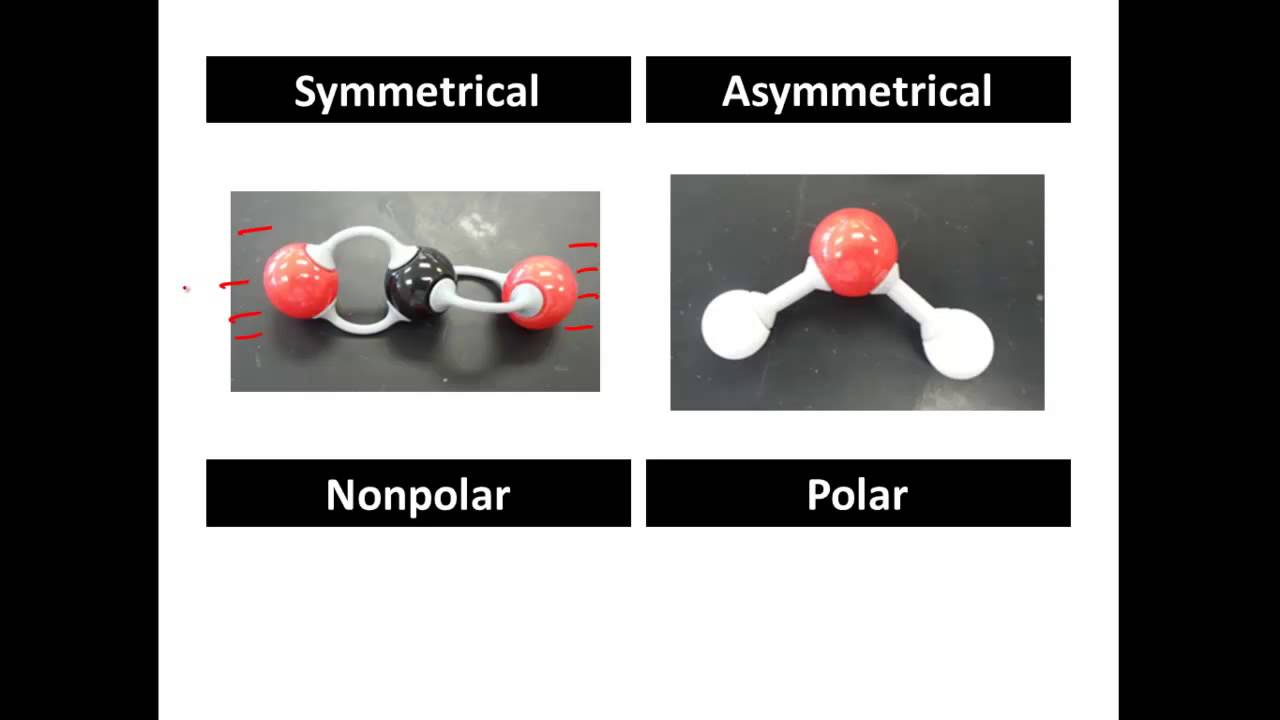
. Electrons will be drawn to. Each atom in HCl requires one. This type of covalent bond exists when the electronegativity of combining atoms differs resulting in unequal electron sharing. Polar covalent bonds form when the constituent atoms have an electronegativity difference between 04 and 17.
Polar Covalent Bonds - Dipole Moments Mathematically dipole moments are vectors. Polar Covalent Bond The electrons are unequally shared between two atoms When a chemical bond involves the sharing of a pair of electrons by two atoms in a molecule it is. Covalent bonds are also affected by the electronegativity of the connected atoms which determines the chemical polarity of the bond. Consider the hydrogen chloride HCl molecule.
Lets have a look at some of them. Polar covalent bonds occur when the difference in electronegativity values is small and the bonding electrons are not shared equally. A polar covalent bond exists when atoms with different electronegativities share electrons in a covalent bond. 22 Polar Covalent Bonds.
A covalent bond in which one atom attracts the shared pair of electrons more strongly than does the other atom and thus has a slightly negative charge. These compounds can be molecules or ions. Some polar covalent bond. In other words the electrons spend more time on one side of the.
A polar covalent bond is formed when atoms which are having different electronegativities and share electrons between them. It means that the individual atoms have to be either highly. A polar covalent bond is a bond in which the electron pair is shared unequally between the two bonded atoms. A covalent compound is a chemical compound that is composed of atoms bonded to each other via covalent bonds.
The O-H bond in water H 2 O is also a polar bond the hydrogen atom has a slight positive charge and oxygen atom has been the slight negative charge ie H δ - O-δ. Now we know that a polar bond is formed when an electron pair is shared. A polar bond is a type of covalent bond in which the electrons forming the bond are unequally distributed. A polar covalent bond occurs when atoms are shared unequally in a covalent bond.
Polar Covalent Bond. Two atoms with equal electronegativity will make. This is due to one of the elements having a higher electronegativity than the other. A polar covalent bond is a bond formed when a shared pair of electrons are not shared equally.
 |
| Polar Covalent Bonds Chemistry Study Guide Chemistry Lessons Teaching Chemistry |
 |
| Polar Covalent Bond Definition Is That As Covalent Bond Between Two Atoms Where The Electrons Forming The Bond Are Covalent Bonding Chemistry Chemistry Lessons |
 |
| Difference Between Polar Covalent Bond And Non Polar Covalent Bond Covalent Bonding 11th Chemistry Bond |
 |
| Water Has Both A Hydrogen Bond And A Polar Covalent Bond Hydrogen Bond Covalent Bonding Chemistry Classroom |
 |
| Difference Between Teaching Chemistry Chemistry Classroom Chemistry Lessons |
Posting Komentar untuk "polar covalent bond"